See the difference in frown lines before and after treatment with XEOMIN® (incobotulinumtoxinA) for injection, for intramuscular use.
XEOMIN® Before and After Photos at Maximum Frown
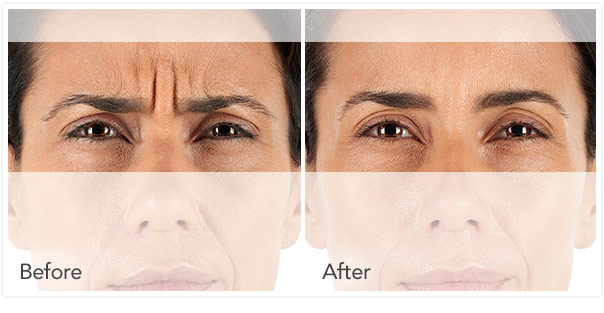 Individual results may vary. Unretouched photos.
Individual results may vary. Unretouched photos.
Claudia, Age 39
Severe Glabellar Frown Lines
The median first onset of XEOMIN® effect occurs within 7 days of injection. Maximum effect occurs at 30 days. The typical duration of effect is up to 3 months, but may last significantly longer or shorter in individual patients. In studies, XEOMIN®-treated subjects ranged from 24 to 74 years of age with an average age of 46.What is XEOMIN®?
XEOMIN® is a prescription medication used in facial aesthetics to temporarily improve the appearance of moderate to severe glabellar frown lines between the eyes (glabellar lines) in adults.
XEOMIN® contains botulinum toxin type A, a protein purified from the bacterium Clostridium botulinum.
XEOMIN® is also sometimes referred to as incobotulinumtoxinA.
XEOMIN® in Aesthetics: How Does it Work?
When you squint or frown, the muscles between your brows contract, causing the skin to furrow and fold. These lines that occur due to facial mimics are referred to as dynamic lines. Over time, as your skin ages and loses some of its elasticity, these repeated contractions can cause persistent frown lines.
Botulinum toxin type A – the active ingredient in XEOMIN® – is used in facial aesthetic treatment to treat dynamic lines like glabellar frown lines. It acts on nerve endings in muscles to prevent muscle fibers from contracting. By reducing these contractions, XEOMIN® can temporarily reduce the frown lines on your forehead between your eyes.
FAQs
IS XEOMIN® PROVEN?
XEOMIN® (incobotulinumtoxinA) for injection, for intramuscular use, was proven effective in two randomized, double-blind, multicenter, placebo-controlled clinical trials of more than 540 adult patients in the treatment of glabellar frown lines. The average age of patients in the study was 46 years. Patients received 20 Units of XEOMIN® and were classified as responders if they had a 2-grade improvement on a 4-point scale as assessed by the physician and patient. Using these criteria, treatment success was higher with XEOMIN®than placebo on day 30 in both studies (60% and 48% vs. 0% for placebo in both studies). Based on these trials, XEOMIN® received FDA approval for aesthetic use in 2011.
HOW DO I KNOW IF XEOMIN® IS RIGHT FOR ME?
If you are an adult with moderate to severe glabellar lines (frown lines between the eyes), XEOMIN® may be right for you. Talk to your doctor to discuss the benefits and risks of treatment.
Do not take XEOMIN® if you are allergic to botulinum toxin or any of the other ingredients in XEOMIN®. You should also not take XEOMIN® if you have had an allergic reaction to any other botulinum toxin product or have a skin infection at the planned injection site.
PLEASE SEE THE XEOMIN® MEDICATION GUIDE AND FULL PRESCRIBING INFORMATION FOR A COMPLETE DISCUSSION OF THE RISKS AND BENEFITS OF TREATMENT.
WHAT CAN I EXPECT DURING TREATMENT?
During treatment, which usually takes about 10-20 minutes, your doctor will inject XEOMIN® (incobotulinumtoxinA) for injection, for intramuscular use, into the muscles in your forehead that cause frown lines between your brows. No anesthesia is required; however, your physician may use a topical anesthetic or cold pack to reduce any discomfort.
WHEN CAN I EXPECT TO SEE RESULTS?
In clinical trials, some patients observed visible smoothing as early as 3-4 days after injection. The median first onset of effect was less than a week. The maximum effect occurred at 30 days. The typical duration of effect was up to 3 months in clinical trials but may last significantly longer or shorter in individual patients.
(click here for more information on XEOMIN)
